New Alzheimer’s drug approved!
With the approval in the US of Leqembi, there are now 2 drugs available which are meant to slow down Alzheimer's, but watch the side-effects.THE US Food and Drug Administration (FDA) has given full approval to a new prescription drug called Leqembi. It’s a treatment (not a cure, and certainly not a miracle cure) of Alzheimer’s disease. It slows down the development of Alzheimer’s in its early stages.
The medicine works by reducing amyloid plaques that form in the brain. These plaques are a feature of Alzheimer’s.
Amyloid refers to tiny deposits of protein. It does not dissolve.
Leqembi has been tested on people with mild symptoms of Alzheimer’s disease. It is unknown if Leqembi is safe and effective at an even earlier stage or at a later stage of Alzheimer’s disease.
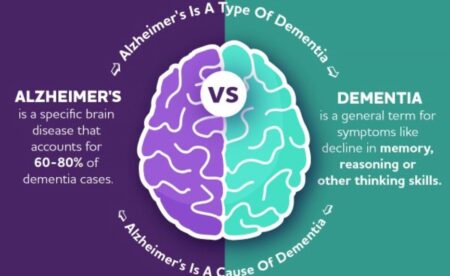
How does Alzheimer’s work?
The disease is named after German pathologist Alois Alzheimer, who identified the plaques and other protein deposits linked to the disease in 1906.
In 1984, a protein fragment called beta-amyloid was identified as the main component of the plaques. Beta-amyloid begins destroying the connections between nerve cells in the brain before it clumps into plaques that lead to nerve cell death. The connections between nerve cells are essential to storing memories, processing thoughts and emotions, and planning and ordering how we move our bodies.
Stopping amyloid deposits in the brain has therefore become the most plausible therapeutic strategy to date.
Leqembi and Aduhelm
Leqembi is one of two FDA-approved prescription drugs to treat Alzheimer’s disease. They aim to delay the formation of amyloid deposits in the brain. In that way, normal brain function should remain intact for longer.
According to Science magazine, hundreds of clinical trials of drugs that would delay or stop the development amyloid plaques in the brain have been conducted.
An article published in the Journal of the Alzheimer’s Association says that developing drugs slowing down Alzheimer’s “remains one of the most difficult therapeutic areas for drug development and has a near 100 per cent failure rate”.
Until Leqembi came along, only a drug marketed as Aduhelm (full name: Aducanumab) was approved by the FDA on the basis that it delayed the development of Alzheimer’s disease. It has not been approved in Australia.
The two main studies of Aduhelm had different outcomes. One found that Aduhelm slowed the progression of the disease compared with people who took a placebo (a treatment containing no active drug).
But in the second study, people who received Aduhelm had disease progression (worsening of symptoms) similar to people who received a placebo. So, on that basis it would be pointless to take Aduhelm.
Until more studies of Aduhelm are completed, it’s not known if the drug is an effective treatment for Alzheimer’s disease. As a result, Aduhelm is marketed under an Accelerated Approval only.
What’s different about Leqembi?
Leqembi had already been approved in January this year, under the Accelerated Approval pathway, the same pathway as for Aduhelm.
An Accelerated Approval pathway allows the FDA to approve drugs for serious conditions where there is an unmet medical need, based on clinical data. In the case of Leqembi, the evidence was the same as it was for Aduhelm: it seemed to reduce amyloid plaques in the brain.
The FDA required the manufacturer of Leqembi to conduct a clinical trial to verify the anticipated clinical benefit of Leqembi.
Leqembi, says the FDA, “demonstrated a statistically significant and clinically meaningful reduction of decline” in this clinical trial. Hence the full approval, which to date has been withheld from Aduhelm.
So, the difference between Leqembi and Aduhelm is that Leqembi works whereas we can’t be sure Aduhelm does.
Both drugs have a long list of quite serious possible side-effects, though.
The most common side effect of both is temporary swelling in areas of the brain, which may cause headache, confusion, dizziness, vision changes and nausea. There are other, less common serious side-effects as well.
The discovery of plaques in the brain by Alois Alzheimer in 1906 has yet to lead to a real break-through both in the identification of what causes Alzheimer’s disease and what will cure it, or at least slow it down.
THE US Food and Drug Administration (FDA) has given full approval to a new prescription drug called Leqembi. It’s a treatment (not a cure, and certainly not a miracle cure) of Alzheimer’s disease. It slows down the development of Alzheimer’s in its early stages.
The medicine works by reducing amyloid plaques that form in the brain. These plaques are a feature of Alzheimer’s.
Amyloid refers to tiny deposits of protein. It does not dissolve.
Leqembi has been tested on people with mild symptoms of Alzheimer’s disease. It is unknown if Leqembi is safe and effective at an even earlier stage or at a later stage of Alzheimer’s disease.
How does Alzheimer’s work?
The disease is named after German pathologist Alois Alzheimer, who identified the plaques and other protein deposits linked to the disease in 1906.
In 1984, a protein fragment called beta-amyloid was identified as the main component of the plaques. Beta-amyloid begins destroying the connections between nerve cells in the brain before it clumps into plaques that lead to nerve cell death. The connections between nerve cells are essential to storing memories, processing thoughts and emotions, and planning and ordering how we move our bodies.
Stopping amyloid deposits in the brain has therefore become the most plausible therapeutic strategy to date.
Leqembi and Aduhelm
Leqembi is one of two FDA-approved prescription drugs to treat Alzheimer’s disease. They aim to delay the formation of amyloid deposits in the brain. In that way, normal brain function should remain intact for longer.
According to Science magazine, hundreds of clinical trials of drugs that would delay or stop the development amyloid plaques in the brain have been conducted.
An article published in the Journal of the Alzheimer’s Association says that developing drugs slowing down Alzheimer’s “remains one of the most difficult therapeutic areas for drug development and has a near 100 per cent failure rate”.
Until Leqembi came along, only a drug marketed as Aduhelm (full name: Aducanumab) was approved by the FDA on the basis that it delayed the development of Alzheimer’s disease. It has not been approved in Australia.
The two main studies of Aduhelm had different outcomes. One found that Aduhelm slowed the progression of the disease compared with people who took a placebo (a treatment containing no active drug).
But in the second study, people who received Aduhelm had disease progression (worsening of symptoms) similar to people who received a placebo. So, on that basis it would be pointless to take Aduhelm.
Until more studies of Aduhelm are completed, it’s not known if the drug is an effective treatment for Alzheimer’s disease. As a result, Aduhelm is marketed under an Accelerated Approval only.
What’s different about Leqembi?
Leqembi had already been approved in January this year, under the Accelerated Approval pathway, the same pathway as for Aduhelm.
An Accelerated Approval pathway allows the FDA to approve drugs for serious conditions where there is an unmet medical need, based on clinical data. In the case of Leqembi, the evidence was the same as it was for Aduhelm: it seemed to reduce amyloid plaques in the brain.
The FDA required the manufacturer of Leqembi to conduct a clinical trial to verify the anticipated clinical benefit of Leqembi.
Leqembi, says the FDA, “demonstrated a statistically significant and clinically meaningful reduction of decline” in this clinical trial. Hence the full approval, which to date has been withheld from Aduhelm.
So, the difference between Leqembi and Aduhelm is that Leqembi works whereas we can’t be sure Aduhelm does.
Both drugs have a long list of quite serious possible side-effects, though.
The most common side effect of both is temporary swelling in areas of the brain, which may cause headache, confusion, dizziness, vision changes and nausea. There are other, less common serious side-effects as well.
The discovery of plaques in the brain by Alois Alzheimer in 1906 has yet to lead to a real break-through both in the identification of what causes Alzheimer’s disease and what will cure it, or at least slow it down.